A System Reaches Chemical Equilibrium When
When a reaction system reaches chemical equilibrium. When a chemical system is at equilibrium ____________________.
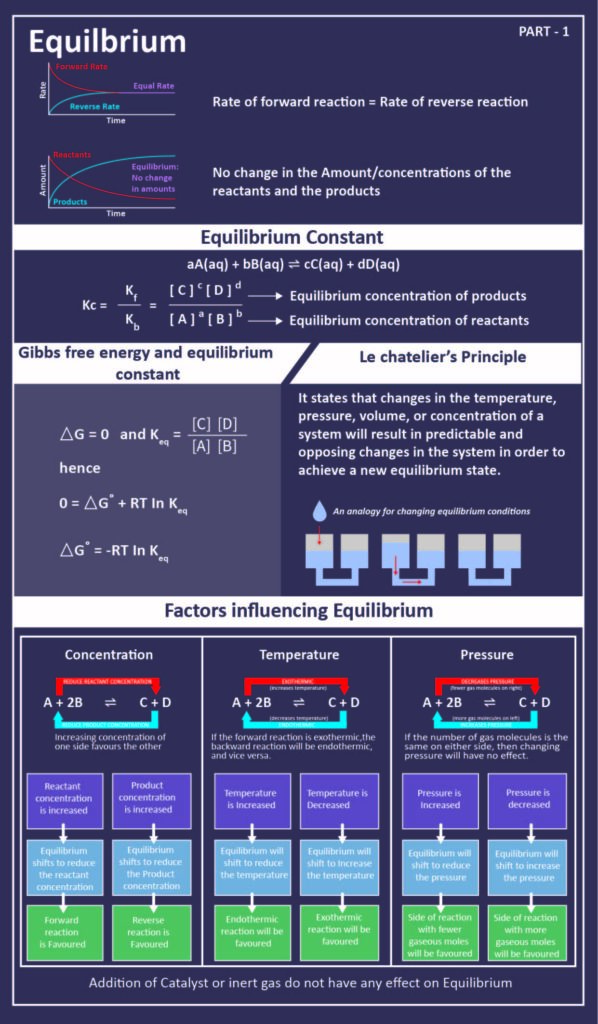
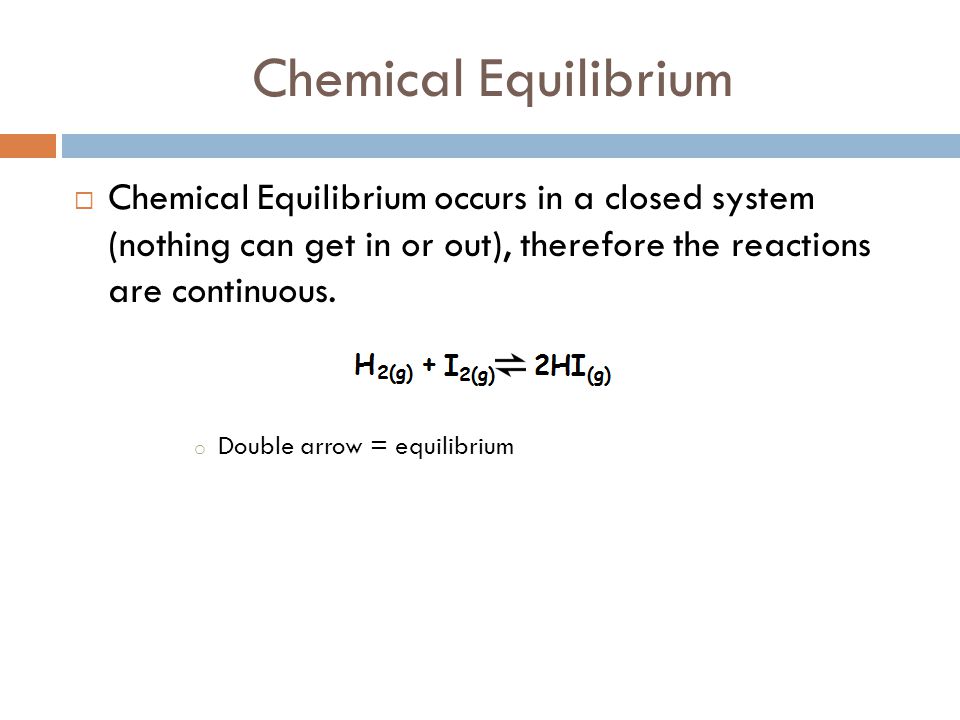
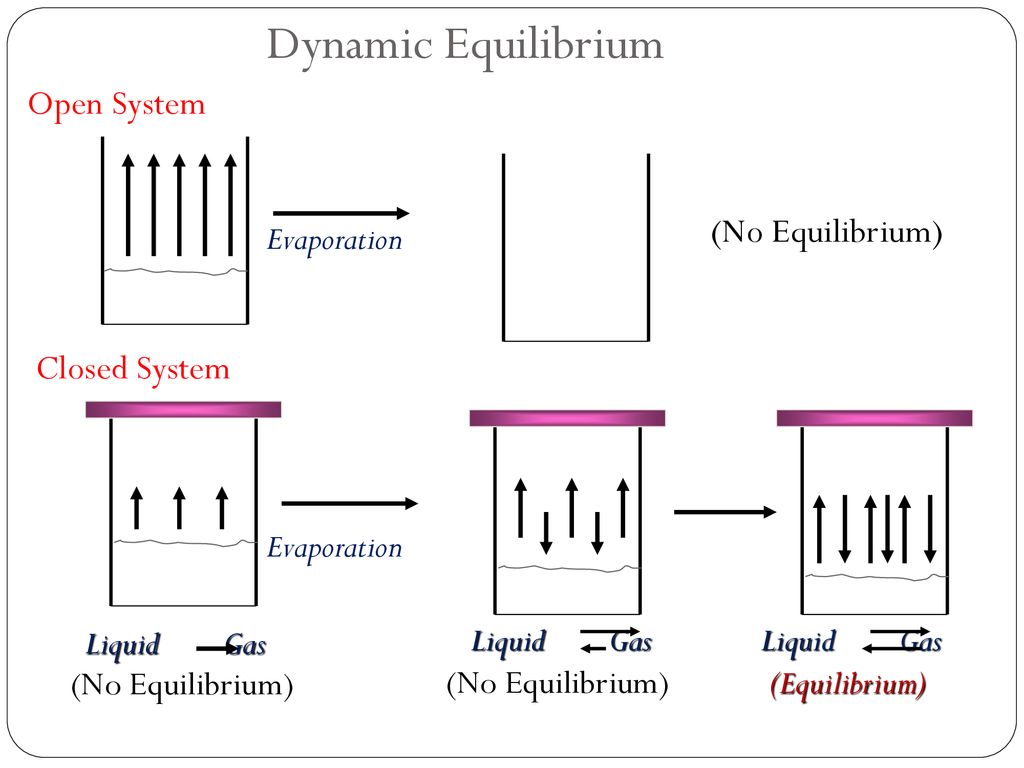
Chemical equilibrium- when a reaction takes place in a closed system it reaches a condition where the concentrations of the reactants and products remain constant over time and the rate of the forward and reverse reactions occur simultaneously and at the same rate.

. A reaction is at equilibrium when the amounts of reactants or products no longer change. Reversible reaction - a chemical reaction that proceeds. Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction.
How would the amount of O2 change when H2 is added to the reaction. The ratio of product concentration to reactant concentration is 11 The reaction quotient is 1 The equilibrium constant is 1 The rate constant of the forward reaction equals the rate constant of the reverse reaction The reaction quotient is. Chemical equilibrium is a dynamic process.
Equilibrium point is the point where the rate of forward reaction is equal the rate of backwards reaction therefore the concentrations of the products and reactants stay the same and do not change once they reach equilibrium. In a closed system it is also possible for a chemical reaction to reach equilibrium. This is because all reactions are.
The condition of chemical equilibrium is reached when. Here in both graphs the forward and reverse reaction are not equal. The rate of consumption of each of the product species by the reverse reaction is equal to the rate of production of each of the reactant species by the reverse reaction.
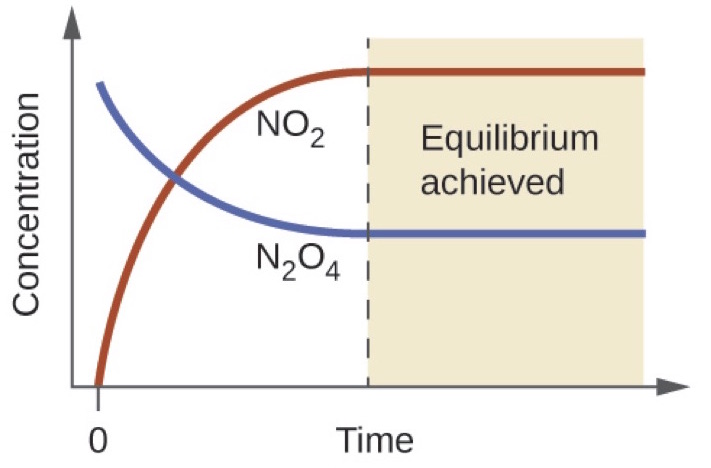
In the table of the decomposition reaction given above the system reached equilibrium first at the time T2 when the concentration of the reactant and products. All chemical equilibria are dynamic equilibria. If a chemical reaction happens in a container where one or more of the reactants.
Can escape you have an open system. A solution at equilibrium is. The forward and reverse reactions continue to occur even after equilibrium has been reached.
CHEMICAL EQUILIBRIUM Section 171 Equilibrium State and Equilibrium Constant Chemical reactions do NOT go to completion 100 products - even those that look like they do. No new product is formed by the forward reaction. The conditions and properties of a system at.
At which time did the system first reach chemical equilibrium. Chemistry questions and answers. For the exothermic reversible reaction below at equilibrium.
A dynamic equilibrium is an equilibrium in which the rates of forward and reverse processes are equal. When there is no further change in the concentrations of the reactants and the products due to the equal rates of the forward and reverse reactions the system is said to be in a state of dynamic equilibrium. The thermodynamic equilibrium occurs when a system reaches the chemical thermal and mechanical equilibriums.
Chemical equilibrium - the state of a reaction in which all reactants and products have reached constant concentrations in a closed system. However because the rates of the reactions are the same there is no change in the relative concentrations of reactants and products for a reaction that is at equilibrium. What happens to the rates of the forward and reverse reactions.
Now we have a rate forward k_fAB and a rate backwards k_rCD. The concentration of reactants in the system is equal to the concentration of products. 2 H2gO2g 2 H2Og a.
Attributions and Licenses This article has been modified from What Is Chemical Equilibrium. Chemical equilibrium is the state in which the forward and backward reaction rates are equal. Chemical equilibrium is the condition which occurs when the concentration of reactants and products participating in a chemical reaction exhibit no net change over time.
A chemical system is considered to have reached equilibrium when. You should note that despite the system being at equilibrium the reaction is still going. Therefore the system cant be in equilibrium.
What happens to the amounts of reactants and products. In this state there is no change in the reactants and products concentrations. A chemical system that has reached equilibrium is one in which the change in the concentration of any reactant or product species is zero Accurate An equilibrium condition occurs when the rate of the forward chemical reaction is equal to the rate of the reverse chemical equation.
The rate of consumption of each of the product species by the reverse reaction is equal to the rate of production of each of the reactant species by the reverse reaction. When the rate of the forward reaction is equal to the rate of the reverse reaction the state of chemical equilibrium is achieved by the system. FeSCN2 aq Fe3 aq SCN- aq In the reaction FeSCN2 ions are red Fe3 ions are pale yellow and SCN- ions are colorless.
Chemical equilibrium is said to be achieved by the system when the rate of the forward reaction is equal to the rate of the reverse reaction. By Siyavula Grade 12 Science CC BY 40. The equilibrium states of a system can be classified as 2.
Reactions instead reach a point ca lled equilibrium after which the amount of reactants and products no longer change with time. Key Concepts and Summary. The reverse reaction no longer occurs in the system.
Use the dissociation reaction to answer the question. That is an opposing reaction occurs in which the products react to a greater or lesser degree to re-form the reactants. When there is no further change in the concentrations of the reactants and the products due to the equal rates of the forward and reverse reactions at the time point of time the system is said to be in a dynamic state of equilibrium.
Group of answer choices. For the generalized equilibrium we have. Here the forward and reverse reaction are equal so from our definition of equilibrium this graphs shows a system in equilibrium.
Chemical equilibrium refers to the state of a reversible chemical reaction in which the rate of forward reaction for the the formation is equal to the rate of backward reaction for the formation of reactants. The sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species. A chemical system is considered to have reached equilibrium when.
The sum of the concentrations of each of the reactant species is equal to the sum of the concentrations of each of the product species. Chemical equilibrium may also be called a steady state reaction. A system reaches chemical equilibrium when a.
Eventually the forward and reverse reaction rates become the same and the system reaches chemical equilibrium the point at which the composition of the system no longer changes with time.

Equilibrium Boundless Chemistry
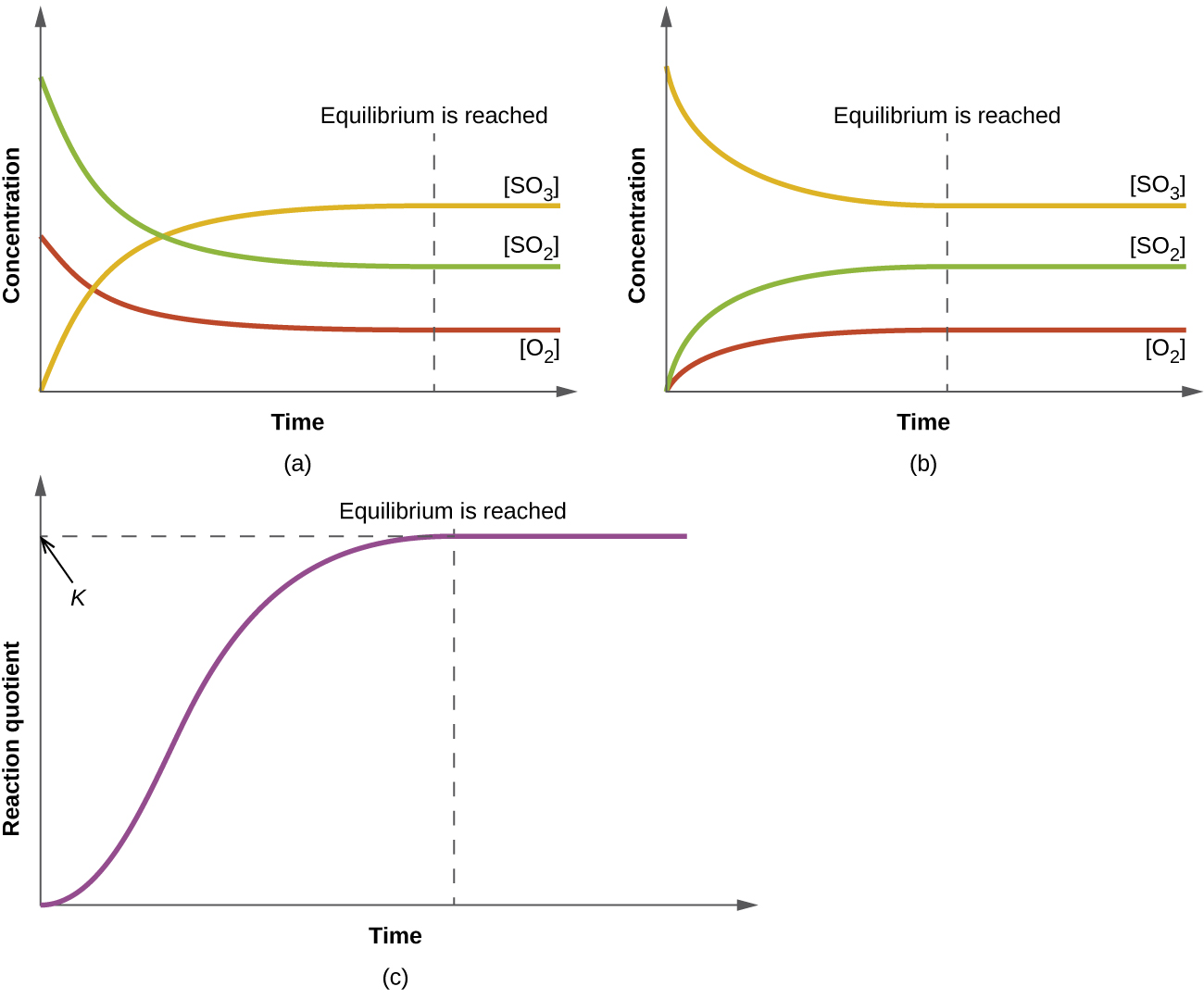
13 2 Equilibrium Constants Chemistry

8 2 Chemical Equilibrium Chemistry Libretexts

8 2 Chemical Equilibrium Chemistry Libretexts

Hsc Chemistry Introduction To Equilibria Science Ready
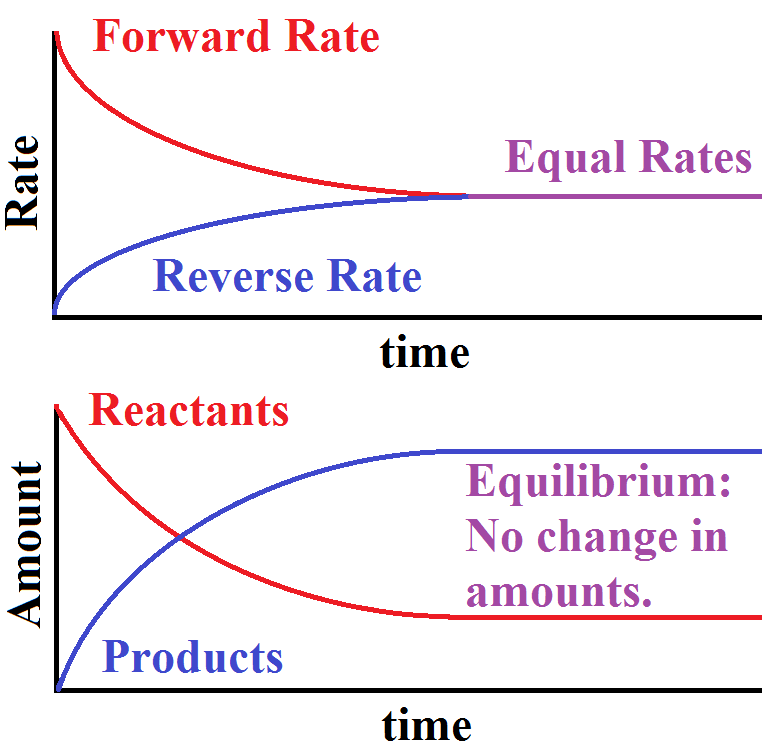
What Determines When A System Reaches Equilibrium What Observations Can Be Made About A System Once Equilibrium Has Been Established Socratic
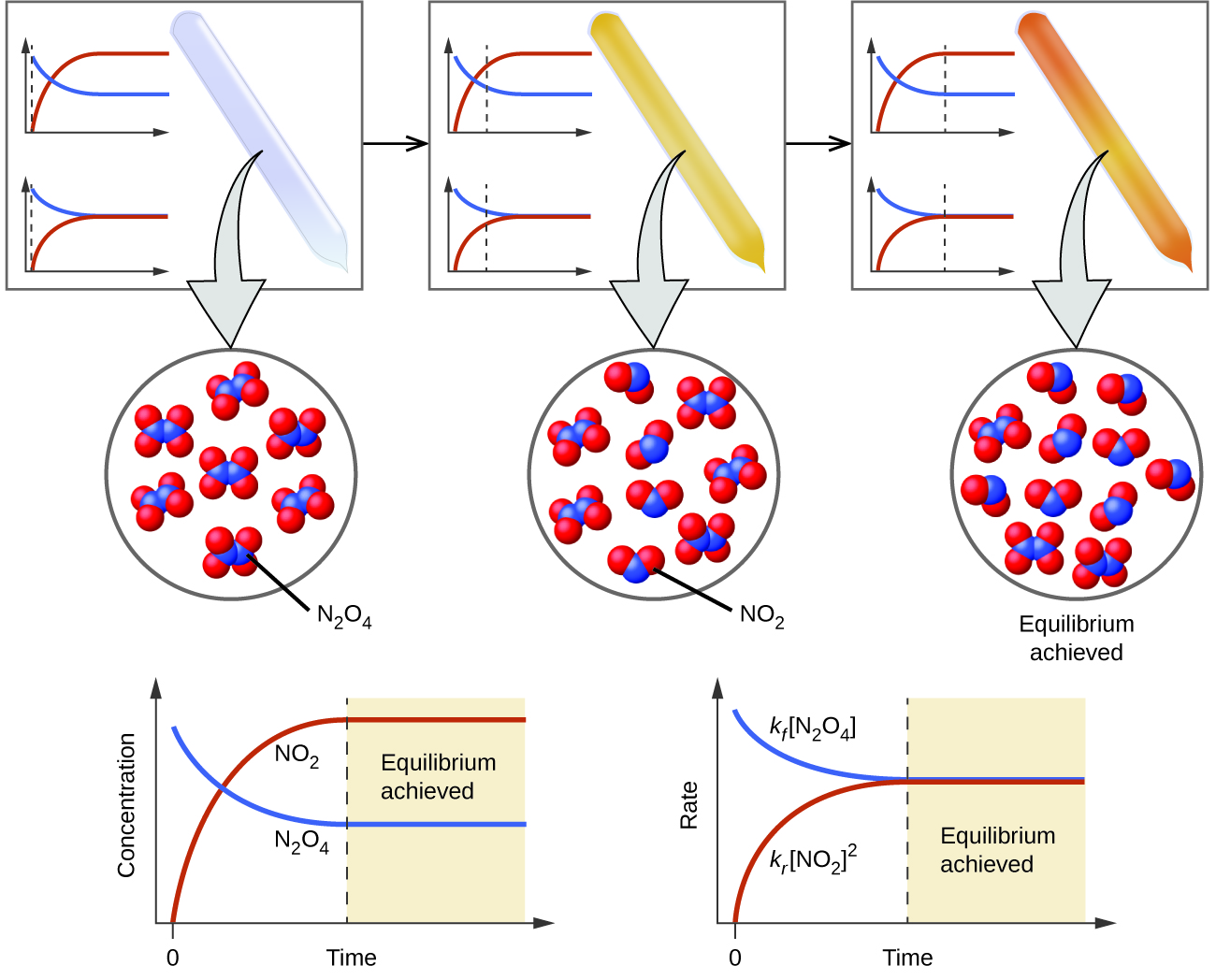
13 1 Chemical Equilibria Chemistry

Equilibrium Boundless Chemistry
Equilibrium And Advanced Thermodynamics Balance In Chemical Reactions Annenberg Learner
The Equilibrium Constant K Article Khan Academy

Ib Topic 7 Equilibrium 7 1 Dynamic Equilibrium Ppt Video Online Download
Equilibrium In Chemical Processes Haber S Process Dynamic Nature Etc

Using The Reaction Quotient To Predict A Pressure Change Worked Example Video Khan Academy

14 1 The Equilibrium State Youtube

Hsc Chemistry Introduction To Equilibria Science Ready
Chemical Equilibrium A Dynamic Process Ppt Video Online Download
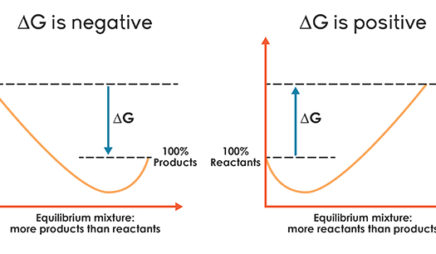
Equilibrium And Advanced Thermodynamics Balance In Chemical Reactions Annenberg Learner
Comments
Post a Comment